Essay
Which of the following compounds is more stable? Explain. 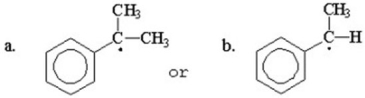
Correct Answer:

Verified
(a)is more stable than (b).Alt...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(a)is more stable than (b).Alt...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q14: Calculate the percentages of each product in
Q15: How many products are formed from the
Q17: An alkane with the molecular formula C<sub>5</sub>H<sub>10</sub>
Q19: Give the structure of the free radical
Q20: Calculate the theoretical percent yields of the
Q21: What is the relative reactivity of 2°
Q21: Which of the following is the best
Q22: Which of the following is the most
Q23: Calculate the percentage of 1-chloro-3,4-dimethylheptane formed in
Q30: How many distinct dichlorination products can result