When 2,2-Dimethyloxirane (See Figure Below)undergoes Anionic Polymerization,the Nucleophile Attacks the Least
Essay
When 2,2-dimethyloxirane (see figure below)undergoes anionic polymerization,the nucleophile attacks the least substituted carbon of the epoxide,but when it undergoes cationic polymerization,the nucleophile attacks the most substituted carbon of the epoxide.Explain why this is so. 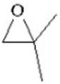
Correct Answer:

Verified
In anionic polymerization,the nucleophil...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q83: What are thermosetting polymers?
Q84: Identify the monomer for superglue.<br>A)acrylamide<br>B)methyl a-cyanoacrylate<br>C)acrolein<br>D)methyl vinyl
Q85: A substituted ethylene polymer in which the
Q86: Which of the following is more likely
Q87: Which of the following statements best describes
Q89: Provide the structure of Saran,an alternating copolymer
Q90: What kind of polymer is produced in
Q91: Which of the following monomers has the
Q92: The first polymer,invented by A.Parke in 1856,was<br>A)Polyethlene.<br>B)Polyester.<br>C)Polystyrene.<br>D)Celluloid.<br>E)Teflon.
Q93: List the two major classes of synthetic