Multiple Choice
Silicon,which makes up about 25% of Earth's crust by mass,is used widely in the modern electronics industry.It has three naturally occurring isotopes,28Si,29Si,and 30Si.Calculate the atomic mass of silicon.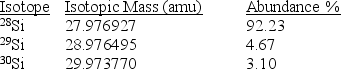
A) 29.2252 amu
B) 28.9757 amu
C) 28.7260 amu
D) 28.0855 amu
E) 27.9801 amu
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Modern studies have shown that the Law
Q9: Which of the following compounds is ionic?<br>A)PF
Q22: For each of the following elements,indicate whether
Q25: What is the name of the acid
Q33: Which one of the following combinations of
Q36: Kaolinite, a clay mineral with the formula
Q38: What is the name of P<sub>4</sub>Se<sub>3</sub>?<br>A) phosphorus
Q63: Ionic compounds may carry a net positive
Q75: Iron (III) chloride hexahydrate is used as
Q80: What is the name of PCl<sub>3</sub>?<br>A) phosphorus