Multiple Choice
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen.
4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 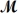
= 26.98 g/mol) and 117.65 g of oxygen ( 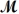
= 32.00 g/mol) is allowed to react.What mass of aluminum oxide ( 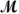
= 101.96 g/mol) can be formed?
A) 155.8 g
B) 200.2 g
C) 249.9 g
D) 311.7 g
E) 374.9 g
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Terephthalic acid, used in the production of
Q19: Balance the following equation: <br>C<sub>8</sub>H<sub>18</sub>O<sub>3</sub>(l) + O<sub>2</sub>(g)
Q26: Lead (II) nitrate is a poisonous substance
Q35: Analysis of a white solid produced in
Q55: Calculate the molar mass of Ca(BO<sub>2</sub>)<sub>2</sub>·6H<sub>2</sub>O.<br>A)273.87 g/mol<br>B)233.79
Q56: Magnesium (used in the manufacture of
Q63: Calculate the molar mass of (NH<sub>4</sub>)<sub>3</sub>AsO<sub>4</sub>.<br>A)417.80 g/mol<br>B)193.03
Q69: One mole of O<sub>2</sub> has a mass
Q70: One mole of methane (CH<sub>4</sub>) contains a
Q76: For a sample consisting of 2.50 g