Multiple Choice
Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron.
3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 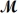
= 24.31 g/mol) and 175 g of iron(III) chloride ( 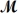
= 162.2 g/mol) is allowed to react.What mass of magnesium chloride = 95.21 g/mol) is formed?
A) 68.5 g MgCl2
B) 77.0 g MgCl2
C) 71.4 g MgCl2
D) 107 g MgCl2
E) 154 g MgCl2
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Alkanes are compounds of carbon and hydrogen
Q12: Phosphine, an extremely poisonous and highly reactive
Q31: Sodium hydroxide, also known as caustic soda,
Q42: When a solution is diluted with water,
Q47: Balance the following equation: <br>B<sub>2</sub>O<sub>3</sub>(s) + HF(l)
Q64: Magnesium fluoride is used in the ceramics
Q71: The number of hydrogen atoms in
Q73: What will be the final volume of
Q78: Gaseous methanol (CH<sub>4</sub>O) reacts with oxygen gas
Q88: When 2.61 g of solid Na<sub>2</sub>CO<sub>3</sub> is