Multiple Choice
Sodium chlorate is used as an oxidizer in the manufacture of dyes,explosives and matches.Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 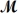
= 106.45 g/mol) .
A) 419 g
B) 169 g
C) 0.419 g
D) 0.169 g
E) 0.00394 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Calculate the mass in grams of 8.35
Q14: Aluminum reacts with oxygen to produce
Q17: What is the mass in grams of
Q23: Hydrochloric acid is widely used as a
Q44: Copper(II) sulfide, CuS, is used in the
Q49: A solution of methanol (CH<sub>4</sub>O) in water
Q51: A 0.150 M sodium chloride solution is
Q55: How many grams of sodium fluoride (used
Q72: Ammonia, an important source of fixed nitrogen
Q84: Potassium dichromate, K<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub>, is used in tanning