Multiple Choice
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, 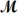
= 139.04 g/mol) .
A) 8.625 M
B) 1.199 M
C) 0.8339 M
D) 0.2031 M
E) 0.008625 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: A compound consisting of C, H, and
Q24: Ammonia will react with fluorine to produce
Q28: The insecticide DDT was formerly in widespread
Q30: What is the percent yield for
Q32: Lithium hydroxide is used in alkaline batteries.
Q36: Calculate the number of oxygen atoms in
Q44: Calculate the number of moles in 17.8
Q66: Balance the following equation for the combustion
Q70: Aluminum metal reacts with chlorine gas to
Q102: Calcium chloride is used to melt ice