Multiple Choice
Predict the products by completing a balanced equation for the following decomposition reaction.
CaCl2(l) 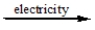
?
A) CaCl2(l) 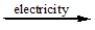
Ca(l) + 2Cl-(l)
B) CaCl2(l) 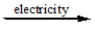
Ca2+(l) + Cl2(g)
C) CaCl2(l) 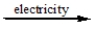
Ca2+(l) + 2Cl-(l)
D) CaCl2(l) 
Ca(l) + Cl2(g)
E) CaCl2(l) 
CaCl(l) + Cl-(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Select the net ionic equation for the
Q16: Which of the following is a weak
Q21: Select the classification for the following
Q35: Select the classification for the following
Q39: In a redox reaction, the oxidizing agent
Q52: Automobile batteries use 3.0 M H<sub>2</sub>SO<sub>4 </sub>as
Q68: a.Explain or define what is meant by
Q70: Select the classification for the following reaction.<br>CaCl<sub>2</sub>·H<sub>2</sub>O(s)
Q103: Which one of the following is a
Q112: In both of the following reactions, a