Multiple Choice
Select the correct set of quantum numbers (n,l,ml,ms) for the first electron removed in the formation of a cation for strontium,Sr.
A) 5,1 ,0,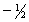
B) 5,1,0,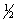
C) 5,0,1,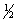
D) 5,1,1,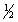
E) 5,0,0,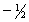
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: With the aid of a diagram,describe how
Q23: Select the most acidic compound from the
Q29: In Mendeleev's version of the periodic table,
Q29: Select the correct set of quantum numbers
Q44: In forming ions of the first series
Q53: Define what is meant by ionization energy,
Q57: The maximum number of electrons in an
Q65: Which of the following ions will be
Q68: Atomic size decreases across a period due
Q100: Elements with _ first ionization energies and