Short Answer
Oxygen difluoride is an unstable molecule that reacts readily with water.Calculate the bond energy of the O-F bond using the standard enthalpy of reaction and the bond energy data provided.
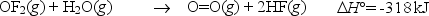

Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF
Q10: In which of these substances are the
Q15: Arrange aluminum,nitrogen,phosphorus and indium in order of
Q17: Which of the following elements is the
Q26: Bond energy increases as bond order increases,
Q36: Electronegativity is a measure of<br>A)the energy needed
Q40: The electrostatic energy of two charged particles
Q42: Select the correct formula for a compound
Q57: Which of the following elements is the
Q74: When an atom is represented in a