Multiple Choice
In which of the following does the nitrogen atom have a formal charge of -1? 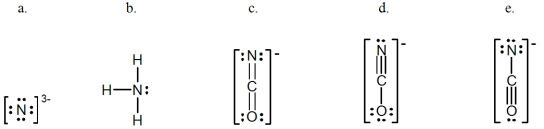
A) a
B) b
C) c
D) d
E) e
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: All possible resonance structures contribute equally to
Q17: The molecule AX<sub>2</sub>, where A and X
Q54: Draw Lewis structures,showing all valence electrons,for:<br>a.N<br>b.Br<sup>-</sup><br><sup> </sup>c.O<sub>2</sub><br><sub>
Q61: Use VSEPR theory to decide which one
Q67: According to VSEPR theory, a molecule with
Q70: According to VSEPR theory, a molecule with
Q76: What is the molecular shape of ClF<sub>2</sub><sup>-</sup>
Q88: Which of the following has no net
Q91: Predict the actual bond angle in SeCl<sub>2
Q98: According to VSEPR theory, a molecule with