Multiple Choice
The formal charges on Cl and O in the structure shown for the ClO- ion are,respectively 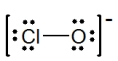
A) 0 and -1
B) -1 and 0
C) 1 and -2
D) -2 and 1
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: In which one of the following species
Q21: Predict the ideal bond angles around carbon
Q21: Use VSEPR theory to decide which one
Q22: Oxygen difluoride is a powerful oxidizing and
Q40: Which one of the following molecules and
Q55: What is the molecular shape of NH<sub>2</sub>Cl
Q62: According to VSEPR theory, a molecule with
Q70: Explain what is meant by "dipole moment",
Q73: Predict the ideal bond angles in IF<sub>2</sub><sup>−</sup>
Q82: According to VSEPR theory, a molecule with