Multiple Choice
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 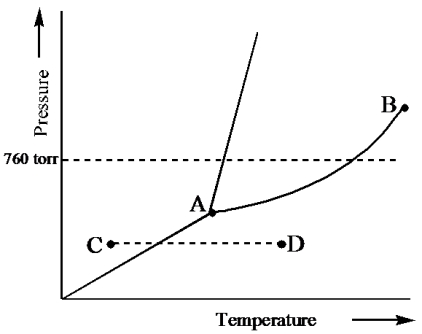
A) increasing temperature with a phase change from solid to liquid
B) increasing temperature with a phase change from solid to vapor
C) increasing temperature with a phase change from liquid to vapor
D) increasing temperature with no phase change
E) increasing temperature beyond the critical point
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Comparing the energies of the following intermolecular
Q10: Which of the following liquids is likely
Q15: Neon condenses due to<br>A)dipole-dipole forces.<br>B)London dispersion forces.<br>C)hydrogen
Q39: A temperature increase causes _ in the
Q43: The energy of a hydrogen bond is
Q52: Which of the following should have the
Q55: For the solid forms of the following
Q68: Hexagonal close packing of identical atoms occurs
Q71: Select the pair of substances in which
Q76: Lead crystallizes in the face-centered cubic lattice.