Multiple Choice
Examine the following phase diagram and determine what phase exists at point F. 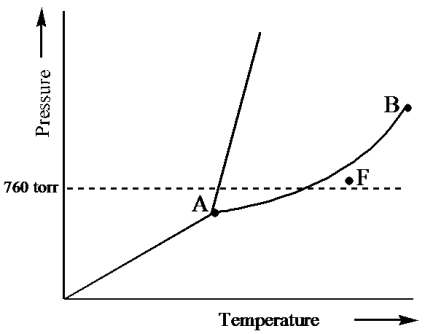
A) vapor + liquid
B) vapor
C) liquid
D) solid
E) supercritical fluid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: In cubic closest packing, the unit cell
Q17: The phase diagram for xenon has a
Q22: Examine the phase diagram for the substance
Q30: Which of the following atoms should have
Q32: In metals, the conduction bands and valence
Q33: A cubic unit cell has an edge
Q55: Assuming that atoms are spherical, calculate the
Q82: The surface tension of water is lowered
Q83: Iron crystallizes in the body-centered cubic lattice.
Q90: A temperature increase causes _ in the