Multiple Choice
Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 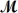
= 86.18 g/mol) at 49.6°C.P°hexane = 400.0 torr at 49.6°C.
A) 54 torr
B) 154 torr
C) 246 torr
D) 346 torr
E) 400.torr
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Potassium hydrogen phosphate is used in the
Q33: Aqueous ammonia is commercially available in a
Q43: Children under the age of six
Q45: Benzaldehyde ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="Benzaldehyde (
Q46: Diethyl ether has a vapor pressure of
Q47: Predict the van't Hoff factor (i)for each
Q53: Isoamyl salicylate ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="Isoamyl salicylate
Q68: Copper(II) bromide is used as a wood
Q77: Octane and nonane are liquids which are
Q89: From the following list of aqueous solutions