Multiple Choice
Safrole is used as a topical antiseptic.Calculate the vapor pressure of a solution prepared by dissolving 0.75 mol of safrole in 950 g of ethanol ( 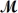
= 46.07 g/mol) .P°ethanol = 50.0 torr at 25°C.
A) 1.8 torr
B) 11 torr
C) 15 torr
D) 40 torr
E) 48 torr
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Raoult's Law relates the vapor pressure of
Q30: Two aqueous solutions are prepared: 1.00 m
Q37: Potassium fluoride is used for frosting glass.
Q38: Ideal solutions do not conform to Raoult's
Q41: If the density of a solution is
Q46: Dimethylglyoxime, DMG, is an organic compound used
Q49: Heats of hydration may be either positive
Q50: What is the molality of a solution
Q66: A 7.112 M solution of sulfuric acid
Q94: If the shell of a raw egg