Multiple Choice
Hexachlorophene is used as a disinfectant in germicidal soaps.What mass of hexachlorophene ( 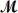
= 406.9 g/mol) must be added to 125 g of chloroform to give a solution with a boiling point of 62.60°C?
Kb = 3.63°C/m,boiling point of pure chloroform = 61.70°C
A) 12.6 g
B) 17.2 g
C) 31.0 g
D) 34.4 g
E) 101 g
Correct Answer:

Verified
Correct Answer:
Verified
Q21: What volume of concentrated (14.7 M) phosphoric
Q27: If a solute dissolves in an endothermic
Q64: The concentration of iodine in sea
Q65: A 0.137 m solution of sodium ferrocyanide
Q69: The Henry's Law constant (k)for carbon
Q71: A solution of sucrose (sugar) in water
Q72: In general, water is a good solvent
Q88: When two pure substances are mixed to
Q96: Determine the freezing point of a solution
Q138: Which of the following aqueous solutions should