Multiple Choice
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 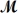
= 74.55 g/mol) in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
A) -1.7°C
B) -0.9°C
C) 0.0°C
D) 0.9°C
E) 1.7°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: For a given solution, which of the
Q9: Saccharin,one of the first non-nutritive sweeteners used
Q15: Select the strongest electrolyte from the following
Q17: Which of the following statements describes the
Q18: Raoult's Law relates the vapor pressure of
Q22: Sodium hydroxide is a common ingredient in
Q74: The solubility of gases in water increases
Q82: Which of the following aqueous liquids will
Q89: The chemist, Anna Lytic, must prepare 1.00
Q90: Which of the following aqueous solutions should