Essay
In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure below shows the concentration of cyclopropane plotted versus time.Use the graph to calculate approximate values of
a.the rate of the reaction,600.seconds after the start.
b.the half-life of the reaction,t1/2. 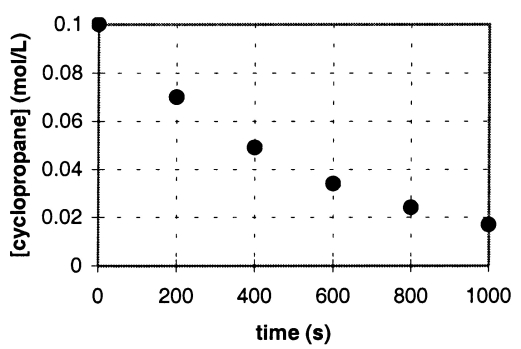
Correct Answer:

Verified
a.Rate = - slope = 6 ± 1  10
10View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q8: The kinetics of the decomposition of dinitrogen
Q17: You are studying the rate of the
Q26: Which of the following sets of units
Q32: The rate of a reaction is determined
Q33: The elementary reaction HBr(g) + Br(g) <font
Q34: A first-order reaction has a half-life of
Q44: For the reaction<br>2A + B +
Q66: The decomposition of SOCl<sub>2</sub> is first-order in
Q68: Briefly list the features/properties common to all
Q77: The rate law cannot be predicted from