Essay
Cyclobutane decomposes to ethene in a first-order reaction.From measurements of the rate constant (k)at various absolute temperatures (T),the accompanying Arrhenius plot was obtained (ln k versus 1/T).
a.Calculate the energy of activation,Ea.
b.Determine the value of the rate constant at 740.K.(In the plot,the units of k are s-1. ) 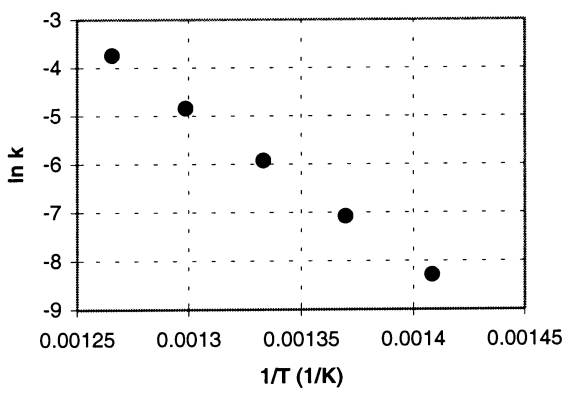
Correct Answer:

Verified
a.260 ± 20 kJ/mol
b....View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
b....
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: A rate constant obeys the Arrhenius
Q2: The gas-phase reaction CH<sub>3</sub>NC <span
Q2: You are required to determine the energy
Q3: What is the molecularity of the
Q4: For the reaction<br>A(g)+ 2B(g) <span
Q5: A reaction has the following rate law:<br>Rate
Q23: Reaction intermediates differ from activated complexes in
Q25: A reaction intermediate is a species corresponding
Q76: Butadiene, C<sub>4</sub>H<sub>6</sub> (used to make synthetic rubber
Q85: Carbon-14 is a radioactive isotope which decays