Essay
A B
At very low pressures many such reactions occur by the following mechanism:
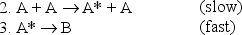
(A* represents a molecule with sufficient energy to overcome the activation energy barrier. )
a.Which of the three reactions above is/are elementary?
b.Where appropriate,identify the molecularity of the reactions.
c.Show that the proposed mechanism is consistent with reaction 1,the observed reaction.
d.Given the mechanism above,suggest a likely rate law for reaction (1).
Correct Answer:

Verified
a.Reactions 2 and 3 are elementary.
b.Re...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
b.Re...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q4: Which one of the following sets of
Q26: The half-life of a second-order reaction does
Q27: Consider the following mechanism for the
Q31: The reaction A <span class="ql-formula"
Q34: A chemical reaction of the general
Q37: Consider the general reaction<br>5Br<sup>-</sup>(aq)+ BrO<sub>3</sub><sup>-</sup>(aq)+ 6H<sup>+</sup>(aq)
Q60: The decomposition of dinitrogen pentaoxide to nitrogen
Q60: When the reaction A <font face="symbol"></font> B
Q64: A reactant R is being consumed in
Q88: The rate constant for the reaction