Multiple Choice
Write the mass-action expression,Qc,for the following chemical reaction.
2Cu2+(aq) + 4I-(aq) 
2CuI(s) + I2(aq)
A) 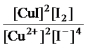
B) 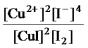
C) 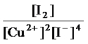
D) 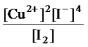
E) 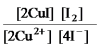
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The two equilibrium constants for the same
Q53: When a reaction system reaches equilibrium, the
Q75: The following reaction is at equilibrium at
Q76: Consider the equilibrium reaction: N<sub>2</sub>O<sub>4</sub>(g) <img
Q77: Ethane can be formed by reacting acetylene
Q79: The equilibrium constant K<sub>c</sub> for the reaction<br>PCl<sub>3</sub>(g)+
Q81: At 25°C,the equilibrium constant K<sub>c</sub> for the
Q82: Hydrogen sulfide will react with water
Q83: The equilibrium constant,K<sub>p</sub>,for the reaction<br>H<sub>2</sub>(g)+ I<sub>2</sub>(g) <img
Q85: What is the mass-action expression,Q<sub>c</sub>,for the following