Multiple Choice
The equilibrium constant for reaction (1) below is 276.Under the same conditions,what is the equilibrium constant of reaction (2) ?
(1) 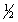
X2(g) + 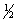
Y2(g) 
XY(g)
(2) 2XY(g) 
X2(g) + Y2(g)
A) 6.02 10-2
B) 7.25 10-3
C) 3.62 10-3
D) 1.31 10-5
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Unless <font face="symbol"></font>H°<sub>rxn</sub> = 0, a change
Q55: Magnesium carbonate dissociates to magnesium oxide and
Q56: At 450°C,tert-butyl alcohol decomposes into water and
Q57: The equilibrium constant,K<sub>p</sub> ,for the reaction<br>CO(g)+ H<sub>2</sub>O(g)
Q58: Consider the reversible reaction: 2NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg"
Q61: For some gas-phase reactions, K<sub>p</sub> = K<sub>c</sub>.
Q61: At 25°C,the equilibrium constant K<sub>c</sub> for the
Q62: A mixture of 0.600 mol of
Q63: At a high temperature,the following reaction
Q64: Write the mass-action expression,Q<sub>c</sub>,for the following chemical