Multiple Choice
A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH.The following data were collected during the titration.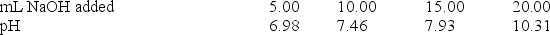
What is the Ka for HClO?
A) 1.1 10-7
B) 3.5 10-8
C) 1.2 10-8
D) 4.9 10-11
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Calculate the solubility of silver oxalate, Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,
Q34: Silver phosphate,Ag<sub>3</sub>PO<sub>4</sub>,is an ionic compound with
Q37: If the pH of a buffer solution
Q39: Which one of the following is the
Q45: A 20.0-mL sample of 0.30 M HBr
Q49: Buffer solutions with the component concentrations shown
Q66: Which of the following substances has the
Q81: A lab technician adds 0.015 mol of
Q89: What is the pK<sub>a</sub> for the acid
Q103: Make a clear distinction between buffer range