Essay
For each of the following pairs,predict which (A or B)will have the greater entropy,and in one sentence indicate your reasoning.
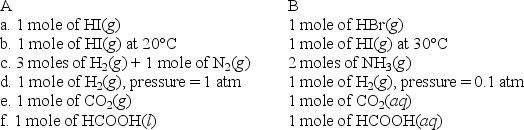
Correct Answer:

Verified
a.A has greater entropy.HI and HBr are c...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
a.A has greater entropy.HI and HBr are c...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q2: The term microstate refers to the energy
Q5: Compare one mole of ice with one
Q33: Under a given set of conditions, all
Q42: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q43: Iron(III)oxide can be reduced by carbon
Q45: Which relationship best describes <span
Q46: You are given pure samples of
Q48: The reaction of methane with water to
Q59: For any reaction, if <font face="symbol"></font>G° >
Q81: For a chemical reaction to be spontaneous