Essay
The complete combustion of liquid benzene is represented by the equation:
C6H6(l)+ 7 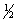
O2(g) 6CO2(g)+ 3H2O(l)
Using the data below,calculate,for this reaction
a. H°
b. S°
c. G° at 25°C.
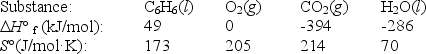
Correct Answer:

Verified
a. H° = -32...
H° = -32...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q33: Under a given set of conditions, all
Q42: In the expression, S = k ln
Q48: The reaction of methane with water to
Q51: The formation constant for the reaction<br>Ag<sup>+</sup>(aq)+
Q52: Elemental boron can be formed by
Q55: A chemical reaction has <span
Q56: What is the free energy change,<sup>
Q58: Which relationship or statement best describes
Q77: Which of the following is always true
Q85: Given: C<sub>2</sub>H<sub>2</sub>(g) <font face="symbol"></font> 2C(graphite) + H<sub>2</sub>(g)