Multiple Choice
Consider the following octahedral complex structures,each involving ethylene diamine and two different,unidentate ligands X and Y. 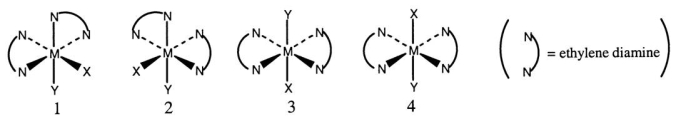
Which,if any,of the following pairs are optical isomers?
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) None of these choices is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: How many unpaired electrons will there be
Q6: All atoms of the first transition series
Q31: Which of the following ligands is most
Q39: Which of the following is considered a
Q47: Why is the +2 oxidation state so
Q51: Which of the following will be paramagnetic?<br>A)V
Q54: Octahedral complexes can exhibit geometric, optical, and
Q68: In the presence of a strong octahedral
Q69: Write the formula for diamminedichloroethylenediaminecobalt(III) bromide.<br>A) [CoCl<sub>2</sub>(en)(NH<sub>3</sub>)<sub>2</sub>]Br<br>B)
Q70: Which of the following should be the