Multiple Choice
Consider the following octahedral complex structures,each involving ethylene diamine and two different,unidentate ligands X and Y. 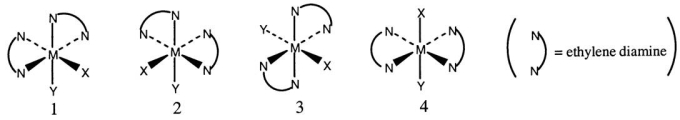
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: The ground state electron configuration of Cr<sup>2+</sup>
Q8: Of the 3d transition series of elements,
Q9: Which of the following species could exist
Q17: The crystal field splitting energy, <font face="symbol"></font>,<br>A)
Q39: Write the formula for sodium tetracyanonickelate(II).<br>A) Na[Ni(CN)<sub>4</sub>]<br>B)
Q40: In the spectrochemical series, which one of
Q44: If M represents a transition element, which
Q49: In the compound [Ni(en)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]SO<sub>4</sub> (where en =
Q60: Tetrahedral complexes can exhibit both optical and
Q61: Apply the valence bond theory to predict