Multiple Choice
Consider the following structures (1 and 2 are octahedral;3 and 4 are square planar) . 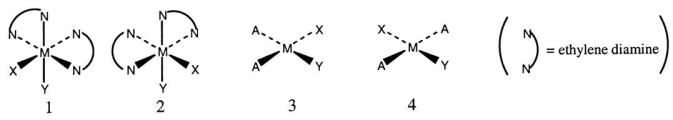
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Give the systematic name for Cr(CO)<sub>3</sub>(NH<sub>3</sub>)<sub>3</sub>.<br>A) chromiumtriaminotricarbonyl<br>B)
Q23: a. State the requirement for two molecules
Q26: a.Explain how the crystal field theory
Q30: Of the 3d transition series of elements,
Q33: The d<sub>xy</sub> and the orbitals d<sub>x</sub><sup>2 </sup>-
Q34: A characteristic of ligands is that<br>A)they are
Q35: A certain transition element has the stable
Q41: Which of the following ligands could participate
Q79: Which of the following will be diamagnetic?<br>A)Ni
Q81: Which of the following ions is most