Multiple Choice
Which of the following statements is false?
A) The limiting reactant is completely consumed in a chemical reaction.
B) The theoretical yield is the amount of product that can be made based on the amount of limiting reagent.
C) The actual yield is the amount of product actually produced by a chemical reaction.
D) The percent yield = 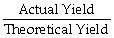
× 100%
E) All of the above are true statements.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Consider the following reaction: 2 Mg +
Q32: The theoretical yield of a reaction is
Q39: How many moles of water are needed
Q53: How many moles of water are made
Q55: How many grams of water are made
Q60: Given the reaction: 2 Na(s)+ Cl<sub>2</sub>(g)→ 2
Q79: What is the limiting reactant for the
Q80: How many grams of water are made
Q87: Iron metal reacts with oxygen to produce
Q101: The theoretical yield is the amount of