Multiple Choice
How much heat (kJ) is needed to raise the temperature of 100.0 grams of water from 25.0°C to 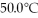
?
A) 10450
B) 0.598
C) 1.05
D) 10.5
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: An energy diagram that shows the reactants
Q35: An example of a chemical change is
Q38: Skim milk is a heterogeneous mixture.
Q49: How would you classify raisin bran?<br>A)pure substance-compound<br>B)mixture-heterogeneous<br>C)pure
Q50: Which of the following items is a
Q52: A 15.0 gram lead ball at 25.0°C
Q61: In physical changes,the atoms or molecules that
Q81: Matter is defined as anything that is
Q90: How many calories are there in a
Q104: The process of boiling water is an