True/False
The correct formula for calculating mass percent of X in compound XY is: 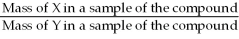
= Mass % X
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: How many moles of iron are contained
Q21: One mole of I<sub>2</sub> has more atoms
Q24: One mole of zinc contains 65.39 zinc
Q25: If you have <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6109/.jpg" alt="If you
Q53: What would the empirical formula be for
Q58: A compound composed of only carbon and
Q61: The mass of one mole of carbon
Q68: One mole of potassium sulfate contains:<br>A)4 moles
Q70: Vitamin C is known chemically by the
Q111: The lighter the atom,the less mass in