Multiple Choice
Which is the excess reactant in the following reaction given that you start with 15.5 g of Na2S and 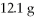
CuSO4?
Reaction: Na2S + CuSO4 → Na2SO4 + CuS
A) Na2S
B) CuSO4
C) Na2SO4
D) CuS
E) not enough information
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: When viewing a chemical equation,the limiting reactant
Q27: Global warming is due to the greenhouse
Q28: Since heat must be supplied to melt
Q66: What is the limiting reactant for the
Q69: Which of the following statements is false?<br>A)The
Q73: Which is the limiting reactant in the
Q75: The actual yield is the amount of
Q75: How many moles of water are needed
Q87: Iron metal reacts with oxygen to produce
Q107: How many grams of the excess reactant