Multiple Choice
How many kJ of heat are needed to completely vaporize 3.30 moles of 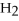
O? The heat of vaporization for water at the boiling point is 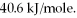
A) 12.3
B) 134
C) 67.0
D) 2.26
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The shapes of protein molecules are determined
Q11: The amount of heat required to melt
Q14: Which substance below is an ionic solid?<br>A)Cu
Q16: Which state of matter has a low
Q17: Substance A is a molecular compound that
Q66: Intermolecular forces are responsible for:<br>A)the taste sensations.<br>B)the
Q71: Which compound will have the highest boiling
Q80: What are the principal forces holding ice
Q102: Water has a heat of vaporization of
Q114: When you make ice cubes:<br>A)it is an