Multiple Choice
How many kJ of heat are needed to completely melt 95.3 g of copper metal,given that the metal is at its melting point? The heat of fusion for this metal is 13.1 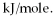
A) 19.6
B) 43.0
C) 1250
D) 0.114
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When sufficient quantity of heat has been
Q26: The melting point is reached when sufficient
Q34: Silicon is which type of solid?<br>A)molecular solid<br>B)ionic
Q44: Which statement about surface tension is FALSE?<br>A)Liquids
Q73: Liquids that are viscous flow more slowly
Q78: Compounds that are similar to water in
Q99: Increasing the intermolecular forces of a liquid
Q104: Gases typically have high densities in comparison
Q113: Atomic solids,such as graphite,have a weak dispersion
Q114: Which molecule below has hydrogen bonding?<br>A)C <img