Multiple Choice
In the following reaction,
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s) :
A) Zn is the reducing agent and CuSO4 is the oxidizing agent.
B) CuSO4 is the reducing agent and Zn is the oxidizing agent.
C) ZnSO4 is the reducing agent and Cu is the oxidizing agent.
D) 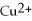
Is the reducing agent and Zn2+ is the oxidizing agent.
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q7: In electrolysis:<br>A)a spontaneous redox reaction produces electricity.<br>B)a
Q13: What is the oxidation state of the
Q15: From the activity list included in this
Q18: From the activity list included in this
Q52: Which of the following are typically TRUE
Q53: It can be shown that copper metal
Q73: Which fact about fuel cells is FALSE?<br>A)Fuel
Q75: Electrons flowing through a wire is an
Q89: Electrical current can be used to drive
Q110: For the reaction KMnO<sub>4</sub> + Li →