True/False
Consider the following sequence of reactions. 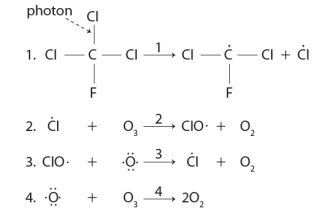 The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Consider the following sequence of four reactions.
Q15: In the past, which of the following
Q16: Which molecule absorbs UV-B radiation?<br>A) O<sub>2</sub><br>B) O<sub>3</sub><br>C)
Q17: What chemical bond is broken by a
Q18: What type of radiation is absorbed by
Q20: In a photochemical reaction,<br>A) the chemical reaction
Q21: Why are scientists worried about the ozone
Q22: Which of the following explains why CFCs
Q23: What does one Dobson unit (DU) equal?<br>A)
Q24: Light with a wavelength of 350 nm