Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Short Answer
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 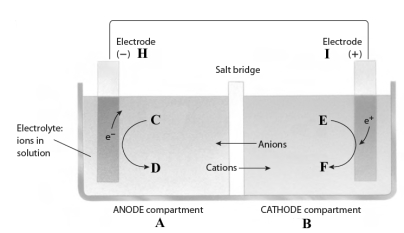
Complete the following questions using the letters shown in the image.
-The electrons flow from electrode _____ to electrode _____.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Identify the reduced species in the
Q34: Which of the following equations represents
Q35: In the equation 2 Na(s) +
Q36: Identify the oxidized species in the
Q37: Consider the following reaction.<br>3Fe<sup>2+</sup>(aq) + 2Al(s)
Q39: Reducing agents gain electrons and are oxidized.
Q40: In an electrolysis reaction, oxidation occurs at
Q41: Consider the following reaction.<br>3Fe<sup>2+</sup>(aq) + 2Al(s)
Q42: PbSO<sub>4</sub>, Pb, and H<sub>2</sub>SO<sub>4</sub> are chemicals in<br>A)
Q43: In the equation SnO<sub>2</sub>(g) + 2