Solved
Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Short Answer
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 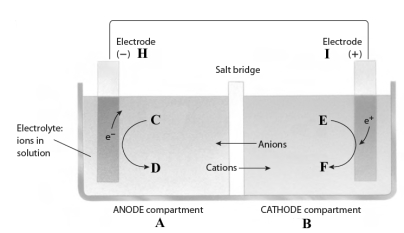
Complete the following questions using the letters shown in the image.
-Al should be placed at letter _____.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: Which of the following is not necessary
Q24: Free radicals which are also oxidizing agents
Q25: Consider the reaction: 3Fe<sup>2+</sup>(aq) + 2Al(s)
Q26: Charge is transported from place to place
Q27: Vitamin C and beta-carotene are antioxidants found
Q29: Which molecule would be a good reducing
Q30: Reduction can be viewed as a either
Q31: In a fuel cell using oxygen
Q32: In a lead storage battery, what lead
Q33: Identify the reduced species in the