Short Answer
Consider the following model of ethanol, CH3CH2OH. 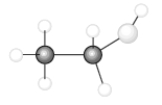 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-The number of carbon to hydrogen single bonds that are broken is_______.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Consider the following model of ethanol, CH<sub>3</sub>CH<sub>2</sub>OH.
Q2: Which process is used to increase the
Q3: The fuel identified as M85 is<br>A)
Q4: Which hydrocarbon would be isolated at the
Q5: Fractional distillation of petroleum<br>A) separates and purifies
Q7: Butane [CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>] and 2-methylpropane [(CH<sub>3</sub>)<sub>2</sub>CHCH<sub>3</sub>] are<br>A) stereoisomers.<br>B)
Q8: A coal gasification process involving a catalyst,
Q9: Which of the following is not true
Q10: Which fossil fuel has a molecular structure
Q11: The hydrocarbon model shown below could represent