Short Answer
Consider the following model of ethanol, CH3CH2OH. 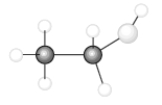 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-During the combustion of one mole of ethanol, the number of O-H bonds formed is _____.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: A compound containing seven carbon atoms could
Q25: Carcinogens are compounds that are<br>A) never used
Q26: The purpose of coal gasification is to<br>A)
Q27: The structure below represents an aromatic hydrocarbon
Q28: Consider the following structure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4139/.jpg" alt="Consider
Q30: Gasohol (E85) contains<br>A) 15% ethanol mixed with
Q31: Which of the bond types shown below
Q32: Petroleum is separated into fractions in the
Q33: What are the bond angles around
Q34: What is the bond angle around