Short Answer
Consider the following model of ethanol, CH3CH2OH. 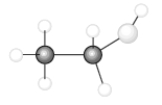 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-Using the data in the following table, 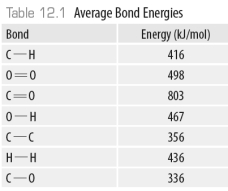 The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
The amount of energy required to break all the C-H bonds in ethanol is ______kJ.
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Which fossil fuel burns cleaner and more
Q45: Identify the alkene shown below.<br>A) C<sub>5</sub>H<sub>12</sub><br>B) C<sub>10</sub>H<sub>20</sub><br>C)
Q46: Which fuel has the largest heat of
Q47: Which is a characteristic feature of alkenes?<br>A)
Q48: Which is a characteristic of alkanes?<br>A) contain
Q50: Which is a representation of cyclohexane?<br>A) <img
Q51: The correct name of the following compound
Q52: Among the alkanes, alkenes, and alkynes only
Q53: The combustible products of most coal gasification
Q54: Which of the bond types shown below