Multiple Choice
Look at the bond energies O-H,N-H,and P-H in the table below.O-H is the hardest bond to break because it has the 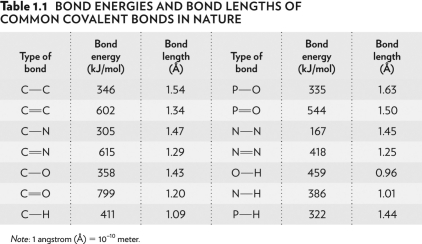
A) greatest difference in relative affinities of the two atoms for electrons.
B) smallest difference in relative affinities of the two atoms for electrons.
C) smallest difference in atomic size.
D) largest difference in atomic size.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Describe the differences between the structures of
Q2: Hydrogen bonds form between hydrogen and<br>A) oxygen.<br>B)
Q3: ATP is an abbreviation for which energy
Q5: The "central dogma of molecular biology" can
Q6: The figure below shows an example of
Q7: If a mutation was made to the
Q8: Amino acids are the building blocks for
Q9: Identify the following biomolecules. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6697/.jpg" alt="Identify
Q10: Who received the Nobel Prize in 1962
Q11: Compare and contrast a transcriptome and a