Multiple Choice
In glycolysis,fructose 1,6-bisphosphate is converted to two products with a standard free-energy change ( 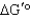 ) of 23.8 kJ/mol.Under what conditions (encountered in erythrocytes) will the free-energy change (
) of 23.8 kJ/mol.Under what conditions (encountered in erythrocytes) will the free-energy change ( 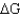 ) be negative,enabling the reaction to proceed to products?
) be negative,enabling the reaction to proceed to products?
A) The free-energy change will be negative if the concentrations of the two products are high relative to that of fructose 1,6-bisphosphate.
B) The reaction will not go to the right spontaneously under any conditions because the 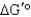 is positive.
is positive.
C) Under standard conditions, enough energy is released to drive the reaction to the right.
D) The free-energy change will be negative when there is a high concentration of fructose 1,6-bisphosphate relative to the concentration of products.
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Which of the following pathways are found
Q59: Which reaction in glycolysis is a redox
Q60: Using the table below,explain why glycolysis is
Q61: A carbohydrate that reacts with oxidizing agents
Q62: Draw the mechanism for bisphosphoglycerate mutase and
Q64: What may be the root cause of
Q65: Fructose 1,6-bisphosphate is converted to two products
Q66: Metabolism is best defined as a collection
Q67: The glycolytic pathway is responsible for passing
Q68: Explain why people who have the genetic