Essay
Use the figure below to answer the following questions. 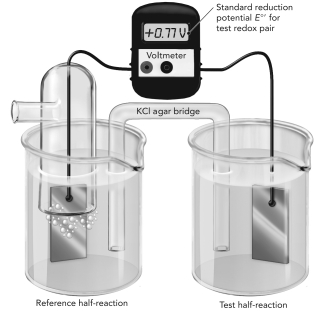 A.The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction.Write each half reaction on the figure and show the flow of electrons.
A.The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction.Write each half reaction on the figure and show the flow of electrons.
B.Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
Correct Answer:

Verified
A.The reference hydrogen half reaction s...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: A patient seeks medical attention for ulcerous
Q64: The primary function of the citrate cycle
Q65: How many ATP are produced from two
Q66: Arsenite affects the lipoamide coenzymes of the
Q67: Which enzyme regulates the flux of acetyl-CoA
Q69: Which molecules function as electron acceptors in
Q70: Compare the two ways the pyruvate dehydrogenase
Q71: In linked metabolic pathways,the oxidants in subsequent
Q72: Which two enzymes in the citrate cycle
Q73: Predict the fate of succinyl-CoA in the