Multiple Choice
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 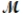 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 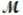 = 32.00 g/mol) is allowed to react. What mass of aluminum oxide (
= 32.00 g/mol) is allowed to react. What mass of aluminum oxide ( 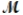 = 101.96 g/mol) can be formed?
= 101.96 g/mol) can be formed?
A) 155.8 g
B) 200.2 g
C) 249.9 g
D) 311.7 g
E) 374.9 g
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Alkanes are compounds of carbon and hydrogen
Q5: Calculate the molar mass of tetraphosphorus decaoxide,
Q34: A compound containing chromium and silicon contains
Q37: A compound of bromine and fluorine is
Q46: Aluminum will react with bromine to form
Q47: Balance the following equation: <br>B<sub>2</sub>O<sub>3</sub>(s) + HF(l)
Q50: Determine the percent composition of potassium dichromate,
Q54: Calcium fluoride, CaF<sub>2</sub>, is a source of
Q55: How many grams of sodium fluoride (used
Q64: Magnesium fluoride is used in the ceramics