Multiple Choice
Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 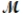 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 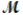 = 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
= 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
A) 68.5 g MgCl2
B) 77.0 g MgCl2
C) 71.4 g MgCl2
D) 107 g MgCl2
E) 154 g MgCl2
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Aluminum oxide, Al<sub>2</sub>O<sub>3</sub>, is used as a
Q25: Constitutional (structural) isomers have the same molecular
Q26: Lead (II) nitrate is a poisonous substance
Q31: How many atoms are in a drop
Q33: Balance the equation<br>B<sub>2</sub>O<sub>3</sub>(s) + NaOH(aq) <font face="symbol"></font>
Q35: Analysis of a white solid produced in
Q37: Balance the following equation for partial oxidation
Q47: Terephthalic acid, used in the production of
Q55: Calculate the molar mass of Ca(BO<sub>2</sub>)<sub>2</sub>·6H<sub>2</sub>O.<br>A)273.87 g/mol<br>B)233.79
Q65: Which of the following samples has the