Multiple Choice
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. 3Mg(s) + 2FeCl3(s) 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 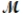 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 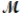 = 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A) Limiting reactant is Mg; 67 g of FeCl3 remain.
B) Limiting reactant is Mg; 134 g of FeCl3 remain.
C) Limiting reactant is Mg; 104 g of FeCl3 remain.
D) Limiting reactant is FeCl3; 2 g of Mg remain.
E) Limiting reactant is FeCl3; 87 g of Mg remain.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What is the mass in grams of
Q7: In combustion analysis, the oxygen content of
Q22: In combustion analysis, the carbon and hydrogen
Q40: Lead(II) sulfide was once used in glazing
Q69: One mole of O<sub>2</sub> has a mass
Q70: In 0.20 mole of phosphoric acid, H<sub>3</sub>PO<sub>4</sub><br>a.
Q71: Ammonia, NH<sub>3</sub>, is produced industrially from nitrogen
Q72: Ammonia, an important source of fixed nitrogen
Q76: For a sample consisting of 2.50 g
Q78: Gaseous methanol (CH<sub>4</sub>O) reacts with oxygen gas