Multiple Choice
Tetraphosphorus hexaoxide ( 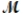 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
A) 57.5%
B) 48.8%
C) 38.0%
D) 32.4%
E) 16.3%
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Sulfur trioxide can react with atmospheric water
Q23: An important reaction sequence in the industrial
Q24: Ammonia will react with fluorine to produce
Q25: The number of hydrogen atoms in 0.050
Q26: Propane, C<sub>3</sub>H<sub>8</sub>, is commonly provided as a
Q28: Balance the following equation: <br>Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s) + SiO<sub>2</sub>(s)
Q31: How many atoms are in a drop
Q47: Terephthalic acid, used in the production of
Q57: Aluminum sulfate, Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>, is used in tanning
Q72: In the combustion analysis of 0.1127 g