Multiple Choice
What is the percent yield for the reaction PCl3(g) + Cl2(g) PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 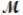 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
A) 195%
B) 85.0%
C) 66.3%
D) 51.4%
E) 43.7%
Correct Answer:

Verified
Correct Answer:
Verified
Q7: In combustion analysis, the oxygen content of
Q15: Terephthalic acid, used in the production of
Q28: Phosphorus pentachloride, PCl<sub>5</sub>, a white solid that
Q38: One mole of methane (CH<sub>4</sub>) contains a
Q40: Lead(II) sulfide was once used in glazing
Q78: Gaseous methanol (CH<sub>4</sub>O) reacts with oxygen gas
Q81: Balance the following equation for the combustion
Q82: Consider the balanced equation for the combustion
Q84: Potassium chlorate (used in fireworks, flares, and
Q87: The iodine "clock reaction" involves the following